Organize your CGM data
Organize your CGM data in the standard format provided
In order to collect and unify data received from various disparate sources, we have a defined a standard format in which you will have to organize your data before you can upload to SFTP. Your CGM data will have to be organized into the following files. The structure is designed considering that this is a research study.Please note that you will have to use the exact same file names as shown below.
Meal and Fitness Data (Optional): Participants may optionally have associated meal and fitness data. Each participant can have multiple files for meals and/or fitness. However, if meal data is provided, corresponding meal metadata must also be included. The same rule applies to fitness data—fitness metadata is required if fitness data is present.The structure is defined below.
View the expected file structure
a. cgm_tracing_0000n.csv
File Description: The “cgm_tracing_0000n” file typically contains a record of continuous glucose monitoring (CGM) data collected over a specific period of time for a patient from various CGM devices. This is the raw CGM data obtained directly from the CGM device.
Note: You can add a suffix of your choice after cgm_tracing to denote the multiple tracing files. The above suggested suffix ” _0000n ” is only a recommendation.
Note: Use comma delimiter in all csv files.
Accepted CGM Data Formats:
The List below provides information about the different types of CGM data sources, along with their respective manufacturers, sensors, and data formats for each platform:
| Manufacturer | Sensors | Data Format (Platform) | File Type |
|---|---|---|---|
| Abbott | Libre2,Libre 3 | Freestyle Libre | CSV |
| Dexcom | G6, G7, Stelo | Clarity | CSV |
| Medtronic | Carelink | CSV | |
| Senseonics | CSV | ||
| Tidepool | any | Tidepool | CSV |
| Glooko | any | Glooko | CSV |
For each patient, a cgm_tracing file containing CGM data can be provided. The metadata associated with each cgm_tracing file can be linked in the cgm_file_metadata file. The number of cgm_tracing files will increase based on the number of patients included in the study.

b. cgm_file_metadata.csv
File Description: Metadata associated with CGM data files. Given below are the columns that provide additional information about the data in the raw cgm_tracing.csv file. Also, a researcher can choose to add custom columns in addition to the columns given below.
| Field | Description |
|---|---|
| metadata_id | A unique identifier for the record |
| devicename | Name of the device |
| device_id | Unique identifier for the device |
| source_platform | Platform or system from which data originated |
| patient_id | Unique identifier for the patient |
| file_name | Name of the uploaded file |
| file_format | Format of the uploaded file (e.g., CSV, excel) |
| file_upload_date | Date when the file was uploaded |
| data_start_date | Start date of the data period covered by the file |
| data_end_date | End date of the data period covered by the file |
| map_field_of_cgm_date | Specifies the column in the file that maps to CGM date time |
| map_field_of_cgm_value | Specifies the column in the file that maps to CGM values |
| study_id | Unique identifier for the study associated with the data |

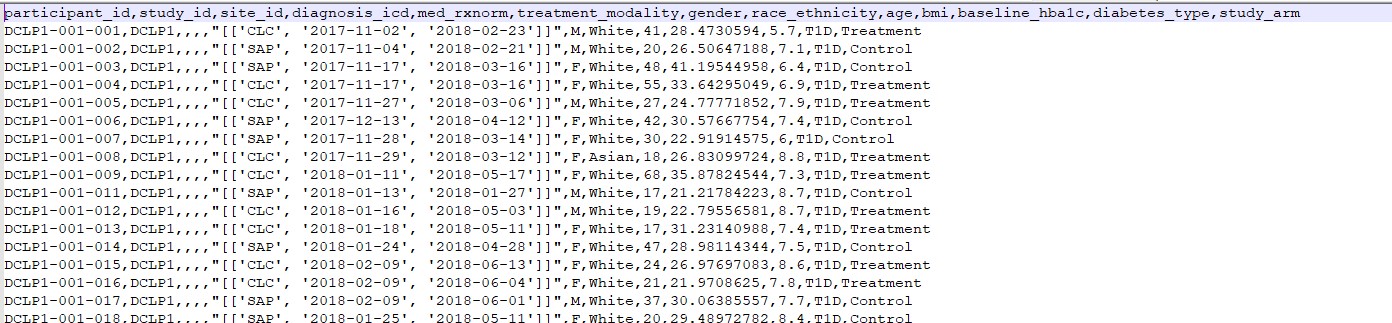
c. participant.csv
File Description: Demographic information of study participants/patients.
| Field | Description |
|---|---|
| participant_id | Unique identifier for the participant/patient |
| study_id | Unique identifier for the study |
| site_id | Identifier for the site where participant is enrolled |
| diagnosis_icd | Diagnosis code based on International Classification of Diseases (ICD) system |
| med_rxnorm | Medication code based on RxNorm system |
| treatment_modality | Modality of treatment for the participant |
| gender | Gender of the participant |
| race_ethnicity | Race and ethnicity of the participant |
| age | Age of the participant |
| bmi | Body Mass Index (BMI) of the participant |
| baseline_hba1c | Baseline Hemoglobin A1c level of the participant |
| diabetes_type | Type of diabetes diagnosed for the participant |
| study_arm | Arm or group to which the participant is assigned in the study |
d. site.csv
File Description: “Site” typically refers to the physical location or locations where the study is being conducted or where participants are recruited. This file shall contain information related to the site in the context of studying CGM data, including details about the specific facilities, clinics, or hospitals involved in the research, as well as any pertinent characteristics or attributes of these locations.
| Field | Description |
|---|---|
| study_id | Unique identifier for the study |
| site_id | Unique identifier for the site |
| site_name | Name of the site |
| site_type | Type or category of the site (e.g., hospital, clinic) |

e. study.csv
File Description: The study file typically contains information about a specific research study.
| Field | Description |
|---|---|
| study_id | Unique identifier for the study |
| study_name | Name or title of the study |
| start_date | Date when the study commences |
| end_date | Date when the study concludes |
| treatment_modalities | Different modalities or interventions used in the study |
| funding_source | Source(s) of funding for the study |
| nct_number | ClinicalTrials.gov identifier for the study |
| study_description | Description about Study |

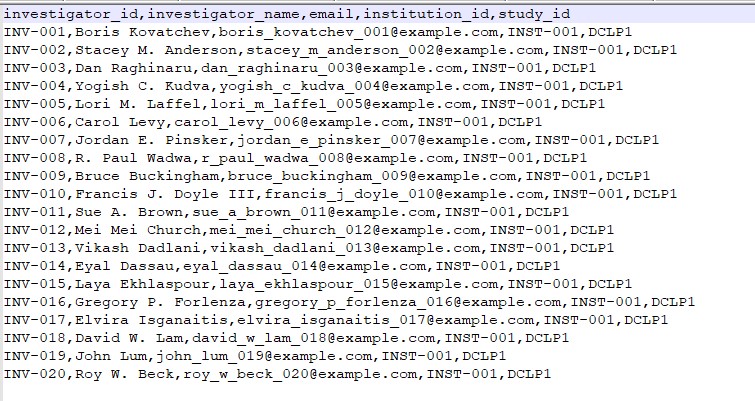
f. investigator.csv
File Description: Details of investigators/researchers involved in the study.
| Field | Description |
|---|---|
| investigator_id | The ID of the investigator / researcher |
| investigator_name | Name of the Researcher |
| Researcher email | |
| institution_id | Unique identifier for the institution |
| study_id | ID for the study associated with the researcher |
g. institution.csv
File Description: This file contains information about institutions involved in a study.
| Field | Description |
|---|---|
| institution_id | Unique identifier for the institution |
| institution_name | Name of the institution |
| city | City where the institution is located |
| state | State where the institution is located |
| country | Country where the institution is located |

h. lab.csv
File Description : This file contains information about laboratories involved in a study.
| Field | Description |
|---|---|
| lab_id | Unique identifier for the laboratory |
| lab_name | Name of the laboratory |
| lab_pi | Principal investigator associated with the lab |
| institution_id | Unique identifier of the institution the lab belongs to |
| study_id | Unique identifier for the study |

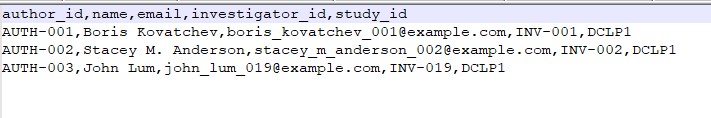
i. author.csv
File Description: This file contains information about authors involved in a study publication.
| Field | Description |
|---|---|
| author_id | Unique identifier for the author |
| name | Name of the author |
| Email of the author | |
| investigator_id | Unique identifier of the investigator the author is associated with |
| study_id | Unique identifier for the study |
j. publication.csv
File Description: This file contains information about publications resulting from a study.
| Field | Description |
|---|---|
| publication_id | Unique identifier for the publication |
| publication_title | Title of the publication |
| digital_object_identifier | Identifier for the digital object associated with the publication |
| publication_site | Publishing site |
| study_id | Unique identifier for the study |

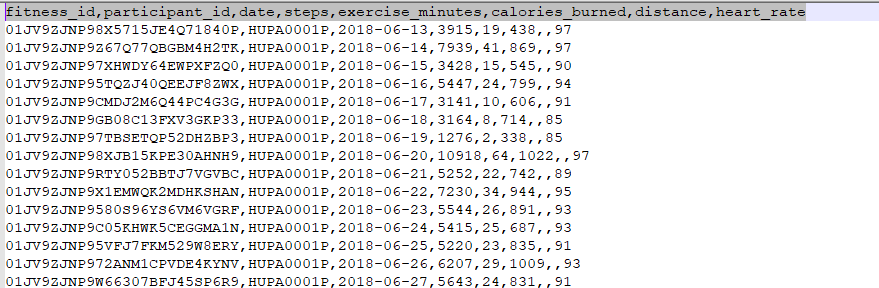
k .fitness_data.csv
File Description: This file contains daily fitness tracking data for participants, including physical activity and biometric measurements.
| Field | Description |
|---|---|
| fitness_id | Unique identifier for each fitness record |
| participant_id | Identifier linking the record to a specific participant |
| date | Date of the recorded fitness activity (YYYY-MM-DD) |
| steps | Number of steps taken by the participant on that day |
| exercise_minutes | Total minutes of exercise performed |
| calories_burned | Number of calories burned during the recorded period |
| distance | Distance covered (in kilometers or miles, depending on context) |
| heart_rate | Average heart rate during the recorded activity period |
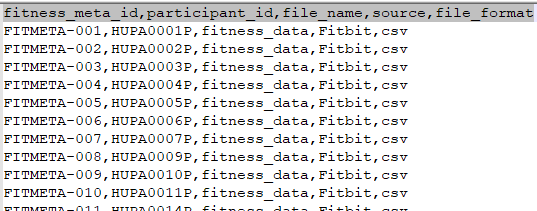
l.fitness_file_metadata.csv
File Description: This file contains metadata about the fitness data files uploaded for each participant.
| Field | Description |
|---|---|
| fitness_meta_id | Unique identifier for the fitness file metadata record |
| participant_id | Identifier linking the metadata to a participant |
| file_name | Name of the fitness data file |
| source | Origin of the file (e.g., device name or app name) |
| file_format | Format of the file (e.g., CSV, JSON) |
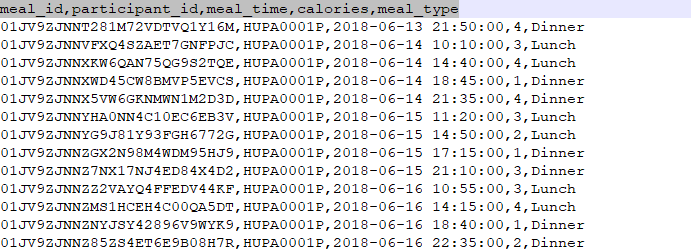
m.meal_data.csv
File Description: This file contains meal intake records for participants, including calorie count and meal type.
| Field | Description |
|---|---|
| meal_id | Unique identifier for the meal record |
| participant_id | Identifier linking the meal to a specific participant |
| meal_time | Timestamp when the meal was consumed |
| calories | Total calories consumed during the meal |
| meal_type | Type of meal (e.g., breakfast, lunch, dinner, snack) |
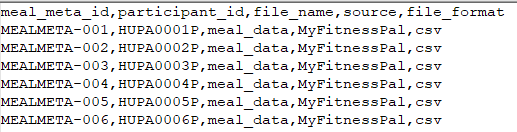
n.meal_file_metadata.csv
File Description: This file contains metadata about the meal data files uploaded for each participant.
| Field | Description |
|---|---|
| meal_meta_id | Unique identifier for the meal file metadata record |
| participant_id | Identifier linking the metadata to a participant |
| file_name | Name of the meal data file |
| source | Origin of the file (e.g., manual entry, app, or device) |
| file_format | Format of the file (e.g., CSV, JSON) |